How To Draw Molecular Orbital Diagram For Co2
Steps to draw hybrid orbital diagrams for a molecule step 1: Read through the provided information, and sketch the lewis dot diagram of the provided compound.

Mo Diagram Of Co2 Preparation Of Gate Csir Netusetset Exam - Youtube
If you have “n” number atomic orbitals, you will form “n” number molecular orbitals

How to draw molecular orbital diagram for co2. Co molecule has 10 valence electrons,four from carbon atom (2s²2p²) and six from oxygen atom (2s²2p⁴).according to molecular orbital diagram, molecular orbital configuration is given as. The maximum number of electrons allowed in an orbital is #2# , each with opposite spins (pauli's exclusion principle). In molecules, electrons are present in the new orbitals called molecular orbitals.
We will also calculate their bond order and determine if t. Molecular orbital diagram for the molecule oxygen o2. Assign x, y, z coordinates (z axis is principal axis;
Click within the orbital to add.may 09, · this feature is not available right now. Draw a picture of the levels. Only 2s and 2p orbitals on c are used in bonding.
First of all mot theory is applicable only for diatomic molecules so mo diagram for co2 is not possible to draw. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at the bottom with the. To view a model, click on an orbital in the energy level correlation diagram shown mouse control of models.
Hydrogen | fluorine | nitrogen | hydrogen fluoride | carbon monoxide | methane | ammonia | ethylene | acetylene | allene | formaldehyde | benzene The purpose of mo theory is to fill in the gap for some behavior that. In picture 2 we show the overlapping p orbitals, which form the bond between the two fl uorine atoms, in red and green gradients.
Watch the video solution for the question: Molecular orbitals for larger molecules 1. In contrast to vsepr and valence bond theory which describe bonding in terms of atomic orbitals, molecular orbital theory visualizes bonding in relation to molecular orbitals, which are orbitals that surround the entire molecule.
Only the 2p orbitals on the two o. Each carbon has 4 and each hydrogen 1 for a total of 12 electrons. Σ2s² σ*2s² πx² πy² σz² π*x⁰ πy⁰ σ*z⁰.
Ethyne, hcch, is a linear molecule. In this video we will draw the molecular orbital diagrams for diatomic nitrogen, carbon and boron. Each carbon atom makes 2 sigma bonds and has no lone pairs of electrons.
Answer to write orbital diagram for co2+. Can be accommodated in the metal d orbitals. Explore bonding orbitals in other small molecules.
A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (lcao) molecular orbital method in particular. Determine point group of molecule (if linear, use d2h and c2v instead of d∞h or c∞v) 2. Σ z y x σ* x y z construct the molecular orbital diagram for.
The orbital diagram shows how the electrons are arranged within each sublevel. Hybridization explains this observation by proposing that there are four hybridized sp3 orbitals instead of 2 s and 3 p orbitals available for covalent bonding. In picture 1 we show the molecular orbital structure of f2.
When combined to form molecular orbitals the bond order is 1: Electron configurations list the orbitals from lower to higher. Inside the dashed lines are the possible molecular orbitals they are capable of forming.
Compare the bond order in h 2 + and h 2 using the molecular orbital energy diagram for h 2. A molecular orbital diagram for a diatomic molecule (two atoms) always has the same basic pattern. Use the buttons at the top of the tool to add orbitals.
Finally, add the valence electrons to the molecular orbital diagram. Molecular orbitals of the second energy level. Label each level with σ, σ*, π, π*
The dashed lines show the remaining p orbitals which do not take part in the bonding. If we arbitrarily define the z axis of the coordinate system for the o 2 molecule as the axis along which the bond forms, the 2p z orbitals on the. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals.
Right click for menu notes Find the characters of the reducible representationfor the combination of valence orbitals on the outer atoms. Click on the co molecular orbitals in the energy level diagram to display the shapes of the orbitals.
Co in molecules with more than one type of atom, mos are formed from aos that have different energies. Ethyne, sp hybridization with two pi bonds 1. For now, trust me that these rules are handy ones:
In a neutral carbon atom, the #1s# sublevel has one orbital with two electrons with opposite spins, represented by the arrows pointing in opposite directions. The atomic orbitals of nearly same energy and same symmetry can overlap to form molecular orbitals. Draw the orbital diagram for the ion co2+.
Like atomic orbitals, molecular orbitals are also characterized by the set of quantum numbers. Molecular orbital (mo) theory is the final theory pertaining to the bonding between molecules. They probably won’t make sense right now, but i’ll explain them when the time is right.
Now coming to main question it's not actually the potential energy of carbon orbital higher than oxygen it's actually the anitibonding molecular orbital whose potential energy which is higher than bonding molecular orbital because the electrons in it is higher excited or unstable state…. Draw the orbital diagram for ion co 2+. Co2 general mo model starting assumptions 1.
These 8 atomic orbitals lead to 8 molecular orbitals the lowest lying 5 orbitals with their occupations in figure 2. If you want to learn how to draw orbital filling diagrams, you need to follow these handy rules. In the mo approach each carbon atom has four valence orbitals namely a 2s and three 2p.
The rules for orbital filling diagrams.

Molecular Orbital Diagram Of Polyatomic Co2 Molecules - Chemical Bonding Molecular Structures - Youtube
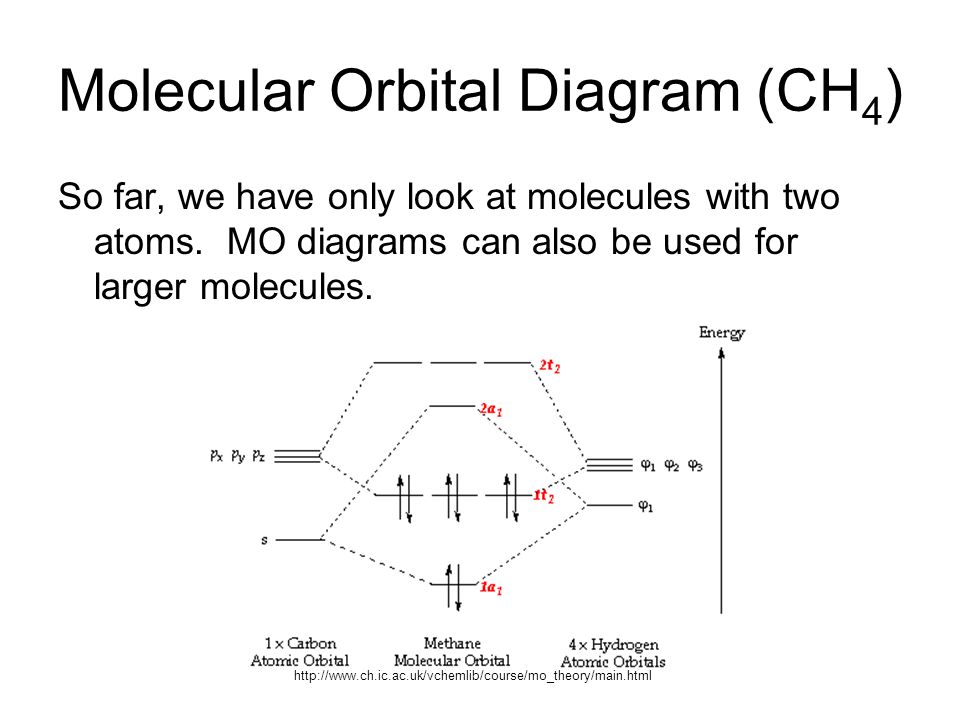
Molecular Orbital Diagram For He2 - Wiring Site Resource

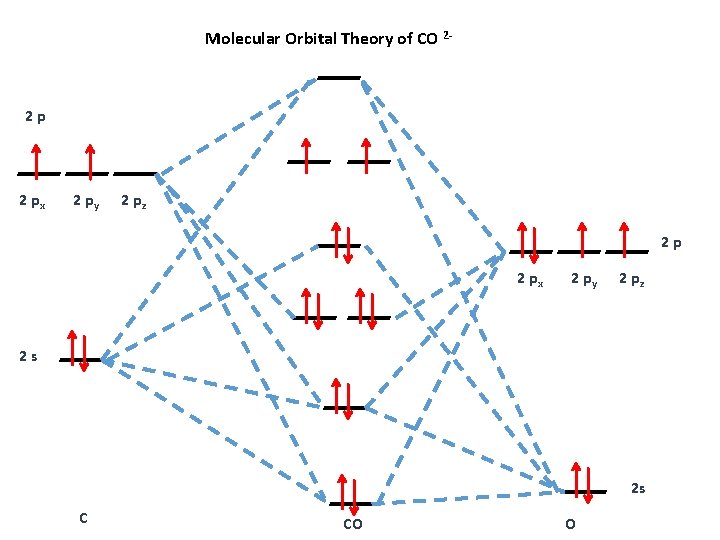
Draw The Mo Diagram For Co2 2- And Identif Clutch Prep

Using The Molecular Orbital Model Write E Clutch Prep

Figure S6 Molecular Orbital Mo Diagram For The Valence Mos Of Ibr Download Scientific Diagram

Pin On Gratitudeabundance Passion

Draw The Orbital Diagram For Carbon In Co_2 Showing How Many Carbon Atom Electrons Are In Each Orbital Studycom

Molecular Orbital Diagram Of Polyatomic Co2 Molecules - Chemical Bonding Molecular Structures - Youtube

Molecular Orbitals For Carbon Monoxide

Molecular Orbital Diagrams In Latex - Tex - Latex Stack Exchange
Carbon Oxides

Molecular Orbital Diagram Of Polyatomic Co2 Molecules - Chemical Bonding Molecular Structures - Youtube
How To Calculate Bond Order From Molecular Orbital Diagram - Wiring Site Resource
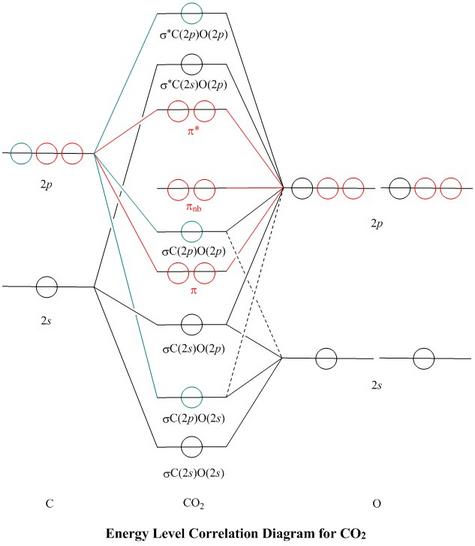
Molecular Orbitals For Co2
Answer In Inorganic Chemistry For Lontum Rodrique 112252
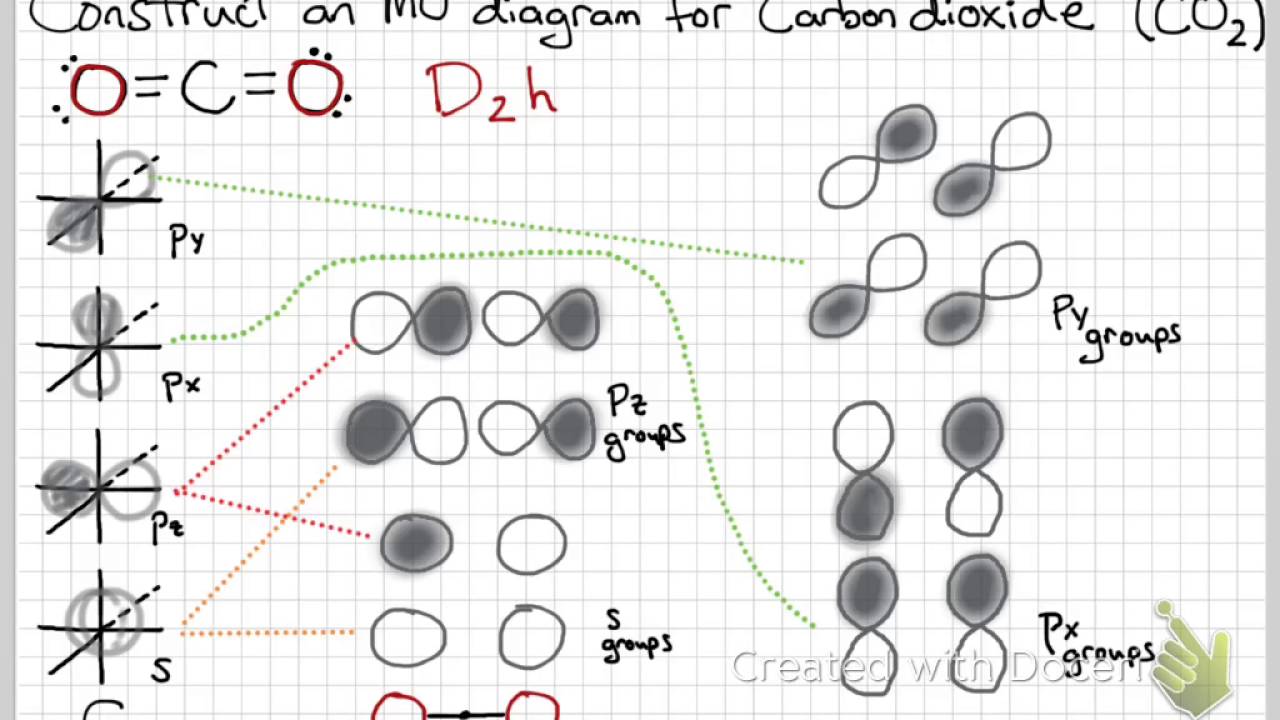
Molecular Orbital Theory Or When Electrons Dont Like
Mo Diagram Co2 - Youtube
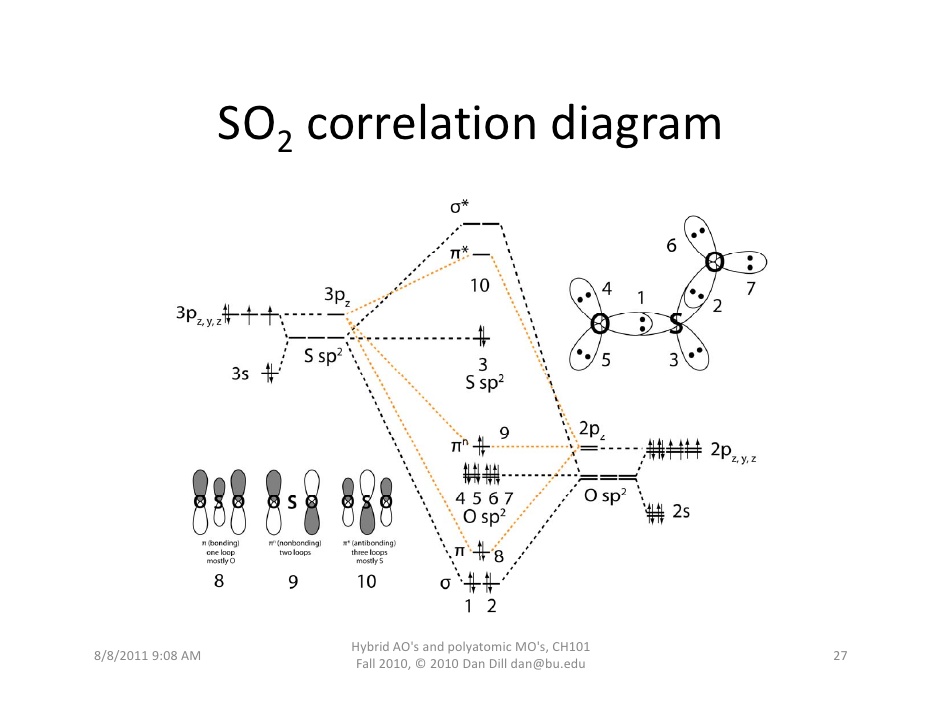
How Do I Correspond The Structure Of So_2 To A Molecular Orbital Diagram Socratic
Draw Mo Diagram Of Co And Calculate Its Bond Order - Sarthaks Econnect Largest Online Education Community